


What is MLD?
Metachromatic leukodystrophy (MLD) is a rare hereditary (genetic) disorder that causes fatty substances (lipids) to build up in cells, particularly in the brain, spinal cord, and peripheral nerves. This buildup is caused by an enzyme deficiency that helps break down lipids called sulfatides. In MLD, the gene and enzyme responsible for this buildup is Arylsulfatase-A (ARSA) and can impact sulfatide buildup in the nervous system, gallbladder, kidneys, and liver. MLD can also be caused by a defect in Saposin B, also referred to as the cerebroside sulfate activator. MLD is caused by variants in the ARSA gene, which codes for the lysosomal enzyme arylsulfatase A. In very rare cases, MLD is caused by mutations in the Prosaposin (PSAP) gene, which codes for the activator protein Saposin B. Although the two genes are different, the symptoms and outcome are the same. The brain and nervous system progressively lose function because the substance that covers and protects the nerve cells (myelin) is damaged.
Presentation and progression can vary widely, depending on the gene variant and at what age the disease presents. Approximately 350 disease-causing ARSA gene variants have been described. Ultimately, MLD causes a decline in motor and cognitive functions as well as severe spasticity, seizures, and increased difficulty moving, talking, eating, and seeing. MLD is estimated to occur in approximately one in every 100,000 live births.
Newly Diagnosed
Inheritance
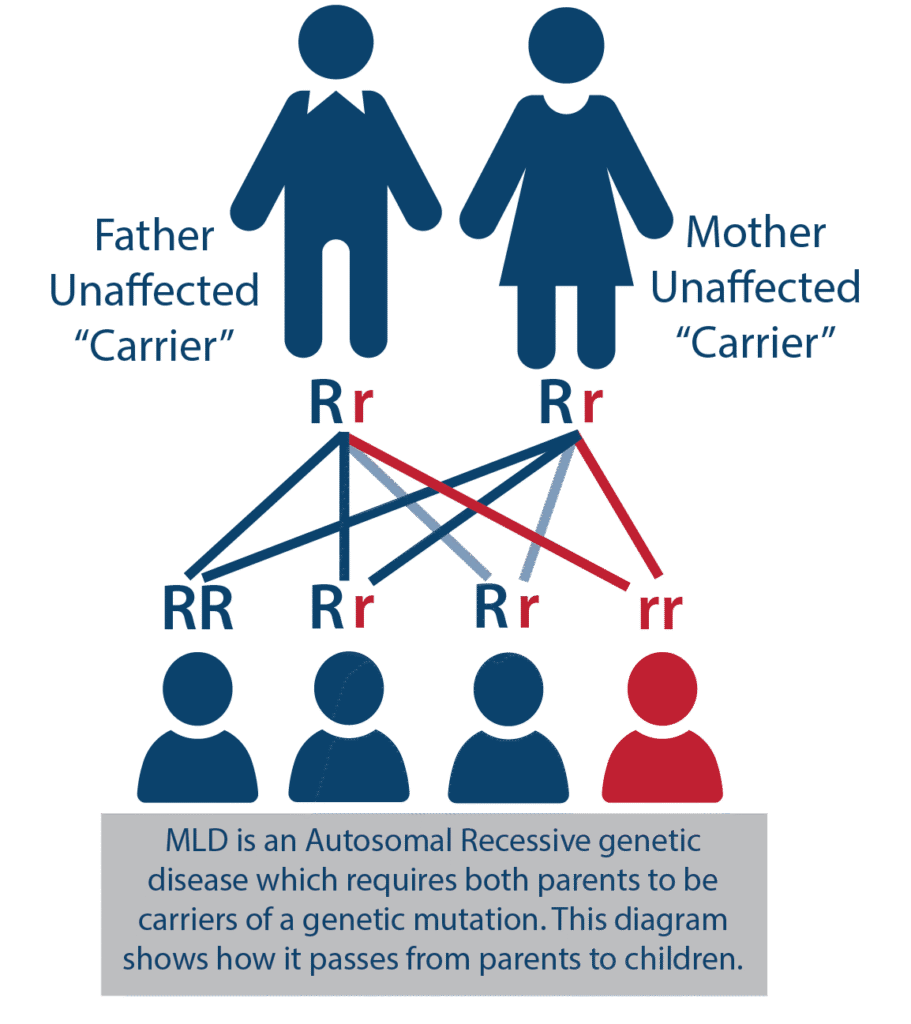
- MLD is inherited autosomal recessively, which means that both parents must be carriers of the genetic variant (clinically healthy). At the time of conception, a child of two carriers has a 25% chance of developing the disease, a 50% chance of being a carrier, and a 25% chance they will not be a carrier of the mutation (visualized below).
- Because the disease can only develop if both parents are carriers, there may not be a family history of the disease, leaving the parents unaware of the potential risks associated with having children.
- When the disease appears, siblings should be tested immediately to determine if they have MLD.
- For each additional child, there is a 25% chance they will inherit both variants and thus suffer from MLD. Members of the wider family circle can also be tested for carriers of the ARSA variant. It is estimated that a single variant occurs in 1 in 100 people.
Clinical Diagnosis
Guidelines for the definitive diagnosis of MLD are based on a combination of medical history, examination, and laboratory findings, including biochemical and genetic testing. (Wang, 2011; Gomez-Ospina, 2024).
A patient with clinical symptoms of progressive neurological dysfunction suggestive of MLD typically receives the following diagnostic tests to confirm the diagnosis:
- ARSA enzyme activity in blood
- Urine sulfatides
- Genetic testing to identify pathogenic ARSA variants
MRIs are also frequently used to determine any white matter changes in the brain associated with leukodystrophies (the group of rare, genetic disorders that impact the white matter of the brain).
References:
Gomez-Ospina N. Arylsulfatase A Deficiency. In: Adam MP, Feldman J, Mirzaa GM, et al., eds. GeneReviews®. Seattle (WA): University of Washington, Seattle; May 30, 2006 [updated 2024 Apr 25].
Wang RY, Bodamer OA, Watson MS, Wilcox WR; ACMG Work Group on Diagnostic Confirmation of Lysosomal Storage Diseases. Lysosomal storage diseases: diagnostic confirmation and management of presymptomatic individuals. Genet Med. 2011;13(5):457-484. doi:10.1097/GIM.0b013e318211a7e1
Forms of MLD

Four clinical subtypes of MLD have been identified:
- Late infantile ≤30 months
- Early juvenile 2.5 – 6 years of age
- Late juvenile 6 – 16 years of age
- Adult MLD >16 years of age
Symptoms
Symptoms of MLD may include developmental delays in children, difficulty walking, progressive loss of skills, changes in behavior, intellectual disability, muscle spasticity, pain, and seizures. In general, the earlier the onset, the more rapid the progression of the disease is.
Late infantile ≤30 months
Initially, the disease is asymptomatic. For late infantile MLD, the first symptoms usually appear between 6 and 30 months of age and can include difficulty walking, tremors, muscle weakness, and balance problems (motor phenotype). These symptoms are usually unnoticeable and easy to confuse with other, less dangerous disorders. Once clinical symptoms become noticeable, they often appear to progress rapidly over a period of several months, with alternating periods of stabilization and decline.
More obvious symptoms include delayed or impaired gait or sudden, unjustified falls. Subsequently, there is a gradual loss of previously obtained developmental milestones, including the ability to independently position the body, speak, bite, and swallow. Other symptoms that may be encountered are listed below.
- Developmental delay
- Hypotonia: decreased muscle tone
- Esotropia: cross-eyed
- Psychomotor regression
- Clumsiness
- Spasticity
- Nystagmus: abnormal eye movement
- Seizures
- Muscle weakness
- Ataxia: loss of ability to coordinate muscular movement
- Quadriplegia: paralysis from the neck down
- Loss of voluntary functions
Early juvenile 2.5 – 6 years of age and Late juvenile 6 – 16 years of age
In the juvenile subtypes of MLD, generally the first symptoms include motor disturbances or a drop in academic performance. This can present as the child having difficulty following directions or showing behavioral abnormalities (trouble paying attention, finishing tasks, disorganized thinking). Other symptoms with juvenile MLD include difficulties walking, slurred speech, motor regression, and incontinence. As symptoms advance, children may develop seizures, abnormal postures, tremors, and gradual loss of voluntary motor function.
Adult MLD >16 years of age
In older children, or adults, deficits and regression related to cognitive functions or changes in behavior are typically seen first. Frequently, these behavioral issues present as: personality change, trouble paying attention, finishing tasks, decline in school/job performance, disorganized thinking, or hallucinations/delusions. Initial psychiatric diagnoses such as schizophrenia or depression are common. Usually, these cognitive symptoms are then followed by motor regression or dysfunction (loss of ambulation, ataxia, dystonia, incontinence) and seizures. The symptoms progress resulting in neurological disability including both physical and intellectual disabilities.
Please Note: A pediatrician or family doctor is often the first to observe abnormalities of psychomotor development in a child. A patient with suspected MLD should be referred urgently to a pediatric neurologist for diagnosis.
medical Resources

Connect with your physician/care team
Your physicians or medical teams will know the most up-to-date information concerning your treatment options and next steps. Connect with your medical providers for the most relevant information for your MLD journey.
If you are interested in exploring clinical trials available for MLD, Clinical Trials has a comprehensive list. Your physician will know more and be able to guide you towards a plan that makes the most sense for you.
Orchard Therapeutics resources
Gene therapy may be a potential option for your family. Learn more about gene therapy below or at Homepage – Orchard Therapeutics
Newborn Screening (NBS) -
MLD Screening at a Glance

What is Newborn screening (NBS)?
What is Newborn Screening (NBS)?
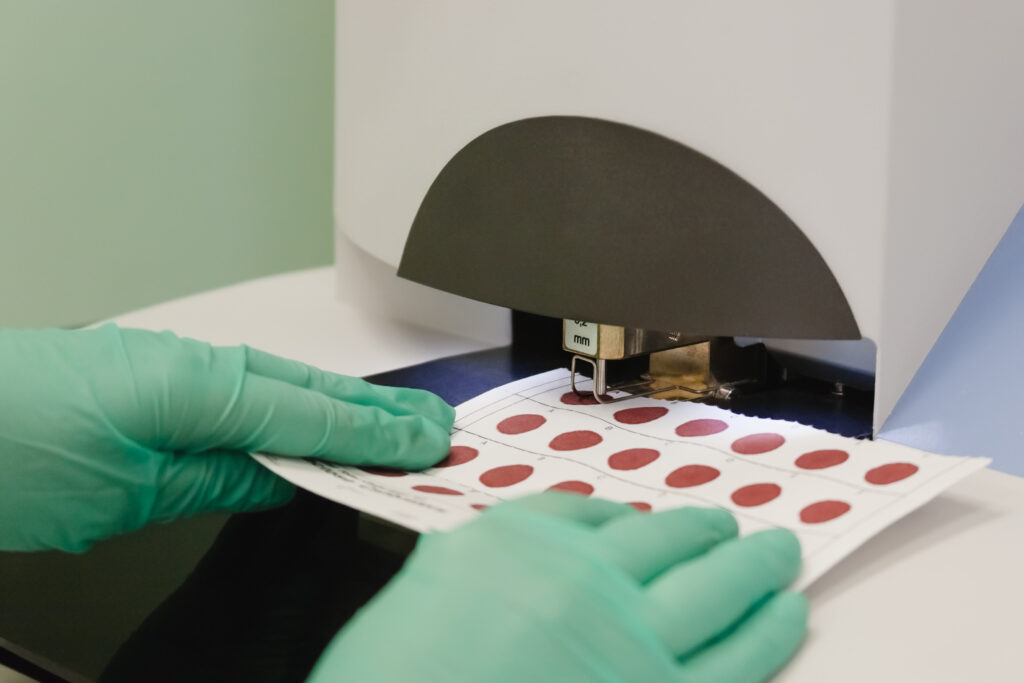
Newborn screening is a way to identify conditions that can impact a newborn’s health and survival. When NBS is performed, the medical teams collect a dried blood spot (DBS) usually from a heel prick, screen the newborns pulse ox, and administer a hearing test. This public health system ensures that children with these core screened conditions have access to lifesaving care and early interventions.
MLD newborn screening is critical for access to care and knowledge about the disease process. Importantly, NBS allows us to detect MLD before symptom onset. This means your loved one can receive early symptom management and intervention to prevent the progression of MLD. Often with rare diseases, a long diagnostic odyssey with multiple misdiagnoses is common. NBS provides a way to limit misdiagnoses and the complex diagnostic journey that families had to go through before. Through NBS, families would be able to know what to expect with their disease progression, therapeutic options, and connect with the MLD community.
How is MLD detected?
With NBS, MLD is detected first through elevated sulfatides in dried blood spots (DBS) from a newborn heel prick, followed by finding low ARSA enzyme activity. A MLD diagnosis is confirmed through genetic testing to sequence the ARSA gene.
Sources:
Where are we headed?

Revvity (formerly PerkinElmer) has their Lab-Developed Test for MLD ready for use and is in the process of FDA approval for Laboratory Developed Tests (LDTs). In the summer of 2024, MLD was also submitted to the Recommended Uniform Screening Panel (RUSP) for consideration and addition to NBS panels across the US.
Treatment Overview
Please talk with your doctor or MLD disease expert to learn what options are available for you or your loved one.
MLD is a rare disease, or “orphan” disease. Currently, no effective treatment is available to reverse the deterioration and loss of function that Metachromatic Leukodystrophy (MLD) causes. To be eligible for commercial gene therapy for MLD, currently, the patient must fall into one of the three diagnostic categories below:
- Pre-symptomatic late infantile MLD
- Pre-symptomatic early juvenile MLD
- Early symptomatic early juvenile MLD
Managing Care
Important steps/reminders:
- Establish a knowledgeable Primary Care Physician (PCP)
- Care team and support system (neurology, gut/nutrition, lungs, heart)
- Symptom management: managing pain, movement dysfunction, occupational and physical therapy, speech therapy
- Late-onset MLD patients may also benefit from behavioral therapy and medications to manage behaviors
- Talk with your team / social worker about medical expenses, funding sources
- Stay informed about clinical trials and treatment options – clinical trials website
- Palliative care: specialized medical care for people with serious conditions aimed at improving the quality of life for both patients and their families. Palliative care can be useful for any stage of a serious condition like MLD, not only when someone is dying, like hospice care. To learn more about palliative care: https://getpalliativecare.org/
- Routine check-ins and evaluations to monitor disease progression and symptomatology
- Children’s Hospital of Philadelphia (CHOP) has a natural history study open to individuals worldwide. To learn more and connect with a study coordinator, check out the MLD natural history page.
Hematopoietic Stem Cell Transplantation (HSCT) / Bone Marrow Transplantation (BMT)
Hematopoietic stem cell transplantation (HSCT), also referred to as bone marrow transplantation (BMT), is available for patients of late onset MLD, but early intervention is essential to be beneficial. This treatment works by introducing cells that produce normal ARSA into the patient to help break down sulfatides. HSCT / BMT may slow the disease progression and thereby increase the quality of life for a patient. Consider this option with your care team, understanding it is most effective in the early or pre-symptomatic stages of the disease in late-onset patients.
New FDA-Approved Gene Therapy
On March 18th, 2024, the U.S. Food and Drug Administration approved Lenmeldy, the first FDA-approved gene therapy indicated for the treatment of children with pre-symptomatic late infantile, pre-symptomatic early juvenile, or early symptomatic early juvenile metachromatic leukodystrophy (MLD). Lenmeldy is a one-time, individualized single-dose infusion made from the patient’s own hematopoietic (blood) stem cells (HSCs), which have been genetically modified to include functional copies of the ARSA gene. Previously in 2020, MLD gene therapy was approved in the EU as Libmeldy. To learn more about the timeline and development of MLD gene therapy, visit Orchard Therapeutics’ website.
The gene therapy for MLD, Lenmeldy or Libmeldy, has been shown to significantly reduce the risk of severe motor impairment, including the inability to walk or sit without support, and death in treated patients compared to untreated patients. Check out Lenmeldy and Orchard Therapeutics to learn more about MLD gene therapy.
Other resources to learn more:
- Katie Snell Presentation
- Family Testimonials for gene therapy from Orchard
