BridgeBio Shares Positive Data from High Dose Cohort of Phase 1/2 CANaspire Study of Gene Therapy BBP-812 for Canavan Disease at ESGCT 2024
–Continued, progressive improvement in motor function and achievement of motor milestones at 12-months post-treatment represents an important and statistically significant change, in contrast to the disease course observed in BridgeBio’s ongoing CANinform natural history comparator study
– Significant and sustained reductions in N-acetylaspartate (NAA) levels in urine, cerebrospinal fluid (CSF), and brain in all participants who received low dose; encouraging trends of further reduction in urine NAA in participants who received high dose BBP-812
– If approved, BridgeBio’s gene therapy for Canavan disease could be the first therapeutic option for children born with this fatal neurodevelopmental disorder

October 24, 2024
Dear Canavan Community,
Aspa Therapeutics, a BridgeBio company, provided an update about the CANaspire investigational gene therapy clinical trial for Canavan disease during a presentation at the 31st Annual Congress of the European Society of Gene and Cell Therapy (ESGCT) in Rome, Italy. Results from the first 11 of the 12 participants who received the investigational therapy, BBP-812, were presented in an invited, peer-reviewed talk by the trial’s lead investigator, Dr. Florian Eichler, M.D., Director of the Leukodystrophy Service at Massachusetts General Hospital, Center for Rare Neurological Disease.
Key Findings:
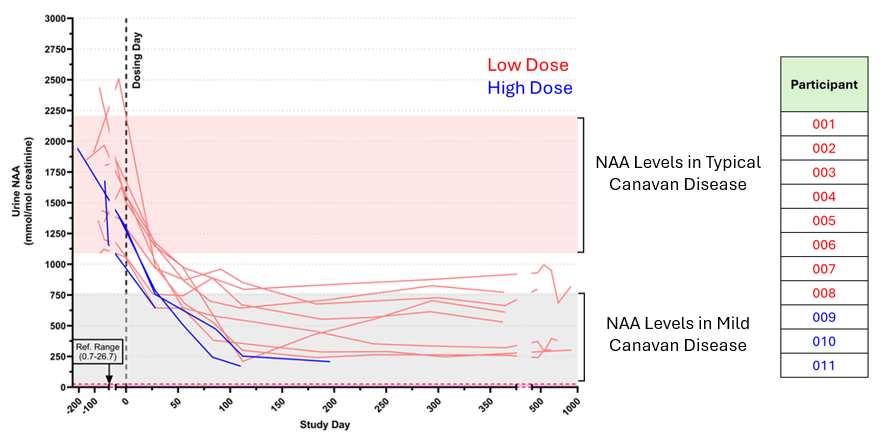
N-acetylaspartate (NAA)
- All participants showed a rapid and lasting decrease in NAA levels—a key chemical marker, which is elevated in children with Canavan disease—after dosing with BBP-812. To date, the average reduction in urine NAA at the low dose was 64.3% and 73.3% at the high dose. An average reduction of 70% in cerebrospinal fluid NAA was also observed. Lower urine NAA levels have been maintained for the duration of the study to date—nearly 3 years in the earliest dosed participants.
- A detailed analysis of published research and natural history data across genotypes of typical Canavan disease and mild Canavan disease demonstrates that the reduced urine NAA levels achieved in study participants are consistent with those seen in children with mild or less severe Canavan disease. The CANaspire study only doses children with typical Canavan disease.
- A reduction in NAA levels is an indicator that the investigational gene therapy may be having a meaningful impact on the disease.
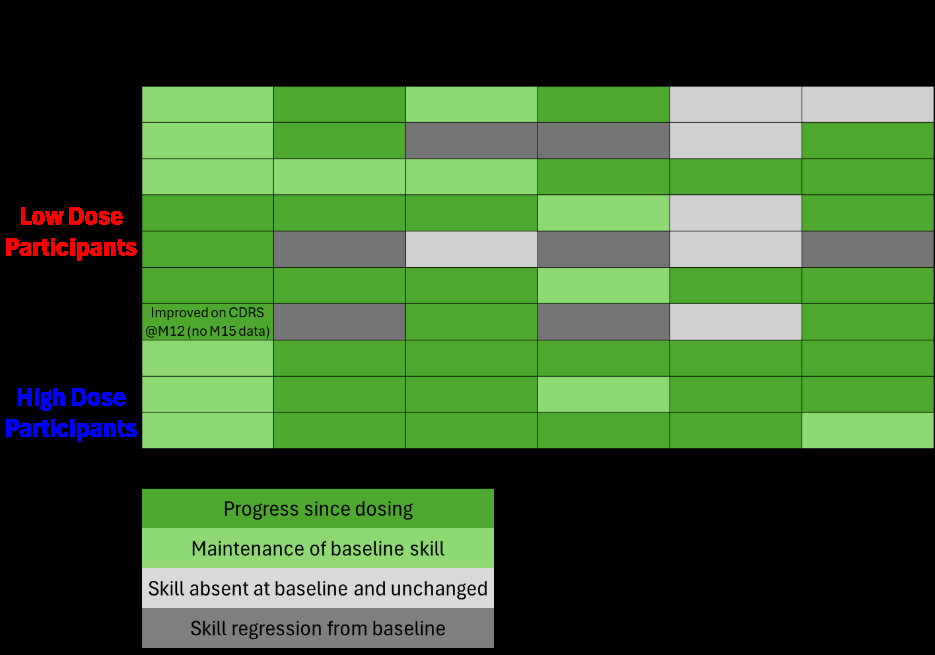
Motor Function
- As mentioned in the press release, all dosed participants have shown post-dose improvements in motor skills or milestones as measured by at least one of several administered developmental tests (GMFM-88, HINE-2, CDC milestones, or the Canavan Disease Rating Scale [CDRS]). This contrasts with the characterized natural history of Canavan disease.
Magnetic Resonance Imaging (MRI): White Matter
- Post-dosing MRI scans have shown improvement in brain myelin, also known as white matter (a type of tissue that is essential for brain function and is impaired in Canavan disease).
Safety
- To date, the safety profile of BBP-812 is consistent with other systemically administered AAV gene therapies.
- All participants experienced at least 1 adverse event (AE); most were mild or moderate in severity and were considered unlikely or not related to BBP-812.
Additional Information
Aspa Therapeutics continues to enroll new participants for the CANaspire gene therapy trial. To be considered for screening for potential participation, a child must meet the following criteria:
- 30 months of age or less at the time of dosing
- Clinical, biochemical, and genetic diagnosis of typical (non-mild) Canavan disease
- Agreement of the clinical investigator(s) on potential eligibility
- Please note: There are additional eligibility criteria that will be reviewed during
Please note: There are additional eligibility criteria that will be reviewed during the screening process.
Updates are available at:
While the data reported here are still early and the final safety and efficacy profile of the investigational gene therapy remains to be fully established, BridgeBio believes these data show the potential of BBP-812. If you have any questions or comments, please reach out to us at canaspire@aspatx.com.
We are grateful for the continued partnership with advocacy organizations and families of the Canavan community as we work together to advance a meaningful therapeutic option for affected children. Special thanks to all the children and families who have participated or expressed interest in participating in this important research.
Sincerely,
The Aspa Therapeutics Team
